| US | EU | Japan | Canada | Taiwan | China | |
|---|---|---|---|---|---|---|
| Regulatory Body 主管機關 | Food & Drug Administration (FDA) | Competent Authority (CA) | Ministry of Health and Welfare (MHW 勞動厚生省) | Health Canada (HC) | National Health Department (NHD 衛生署藥政處) | SFDA (國家食品藥物監督管理局) |
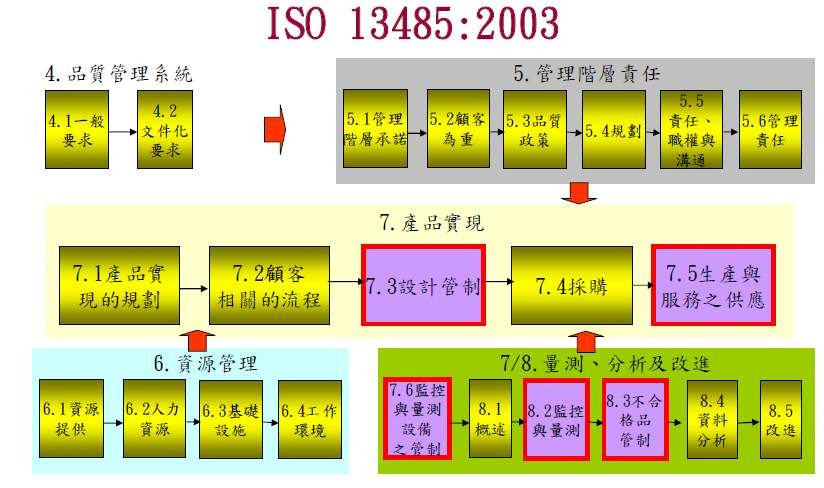
| QS standard依據法規 | 510(k) QSR: 21 CFR 820 | MDD 93/42/EEC ISO13485 | QS standard 1128 | CMDCAS MDR+ ISO 13485 | GMP (ISO13485) | GB (ISO13485) |
| Quality Systems auditor 品質系統稽核 | FDA (Authorized 3rd Body Recognizing) | NB Notified Body (3rd party) | MHW (Authorized 3rd Body Recognizing) | Registrar (Authorized 3rd party) (CMDCAS) | Authorized Body(工研、電檢、金屬、塑膠)、與歐盟NB互認中 | SFDA+ 中國質量認証中心+計量管理局 |
| Pre-market reviewer 上市前審核 | FDA Or 3rd party Review | NB | MHW | HC | AB(GMP)+NHD(查驗登記) | SFDA |
| Post-market compliance & Enforcement 上市後監督及執行 | FDA(Authorized 3rd Body Recognizing) | NB+ CA | MHW(Authorized 3rd Body Recognizing) | HC Authorized 3rd party | AB+NHD | SFDA+ 中國質量認証中心+計量管理局 |
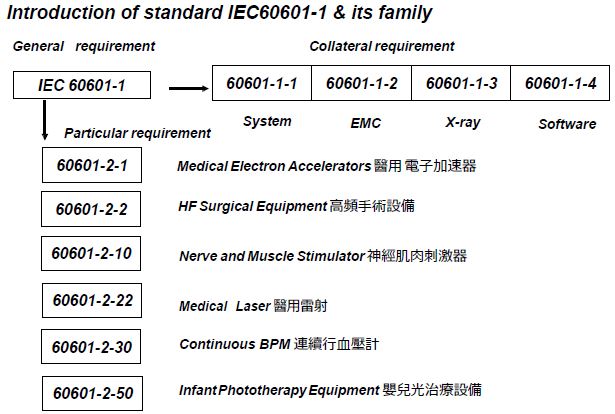
各國廣泛採用的醫療器材標準,包括: